

All products
Dry eye preparation
antibiotics
anti-allergic preparations
Steroids preparation
Glaucoma treatment
retinopathy treatment
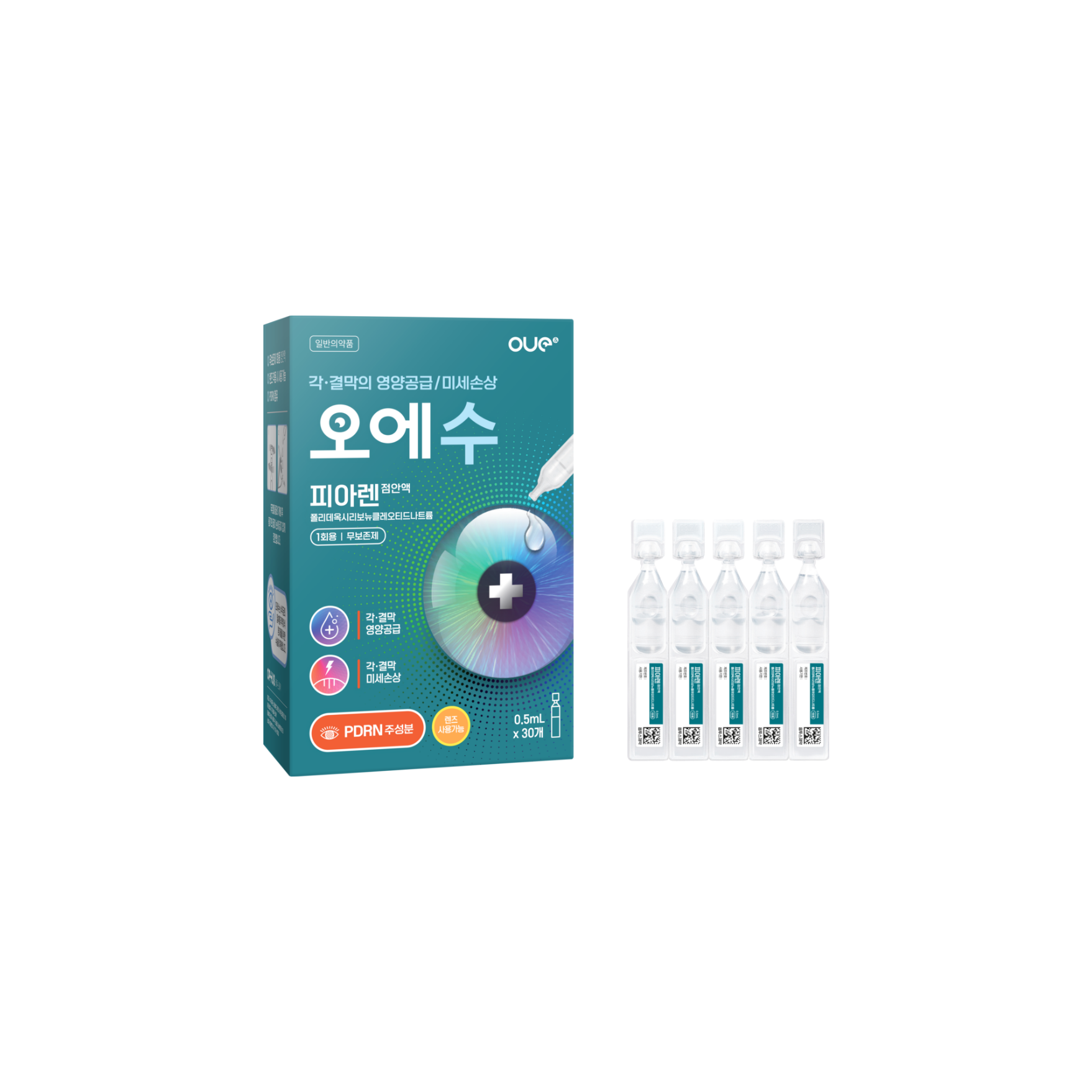
Preservative-free Lubricant Eye drops
(Polydeoxyribonucleotide Sodium)| Classification | |
|---|---|
| Active ingredients |
Polydeoxyribonucleotide Sodium 0.75mg/mL |
| Indications |
Provides nutrition for ulcerative diseases of the cornea and conjunctiva caused by nutritional deficiencies. Addresses micro-damage to the cornea and conjunctiva caused by wearing contact lenses. |
| Dose |
Instill 2-3 drops, 2 to 4 times a day. Discard the remaining liquid and container after use. |
| Packaging Unit | 0.5mL X 30s |
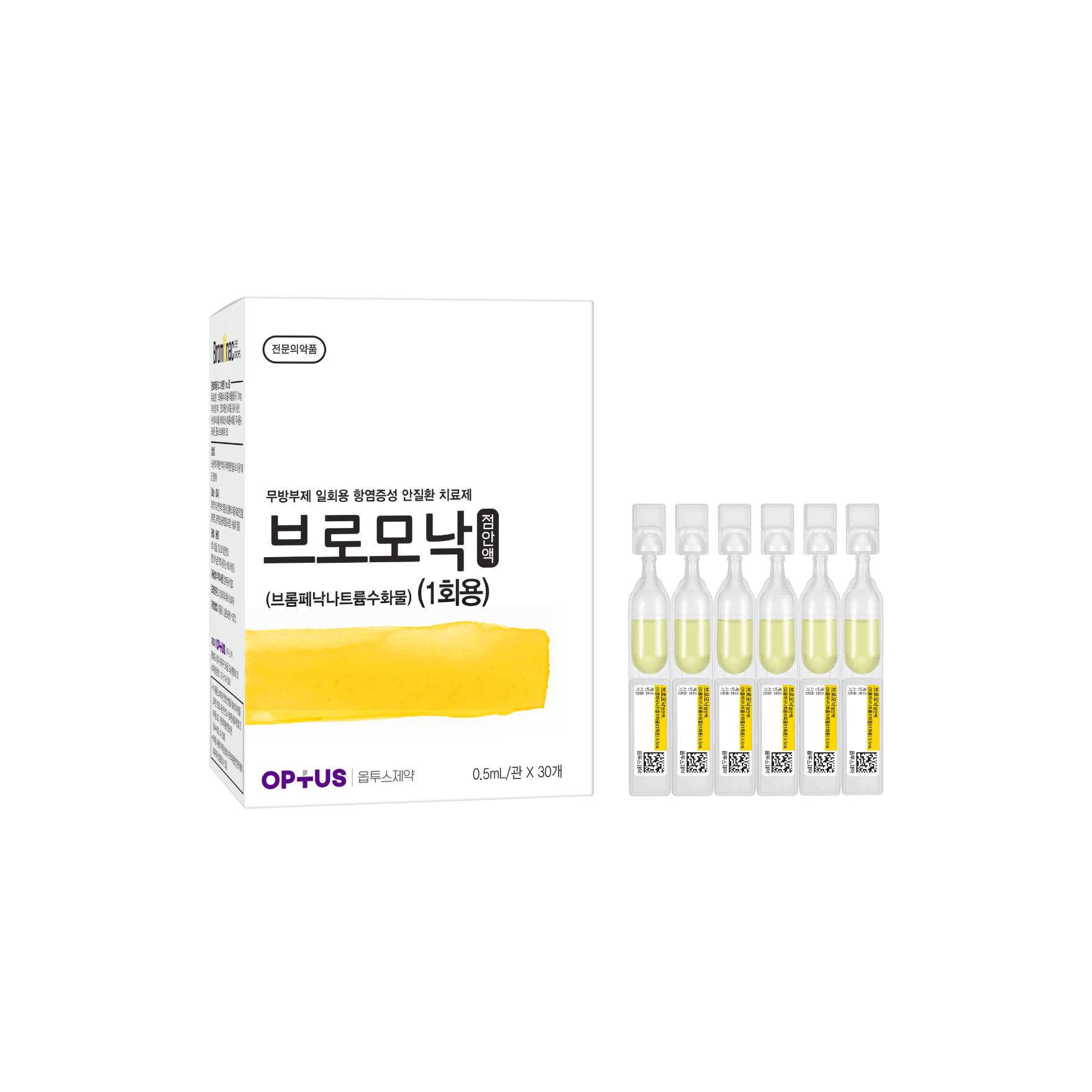
Preservative-free NSAID eye disease treatment
(Bromfenac Sodium Hydrate)| Classification | |
|---|---|
| Active ingredients |
Bromfenac Sodium Hydrate 1.0mg/mL |
| Indications |
Inflammatory diseases of the outer and anterior segment of the eye (blepharitis, conjunctivitis, scleritis (inclUnit Doseing episcleritis), postoperative inflammation) |
| Dose | One drop twice daily |
| Packaging Unit | 0.5mL x 30s |
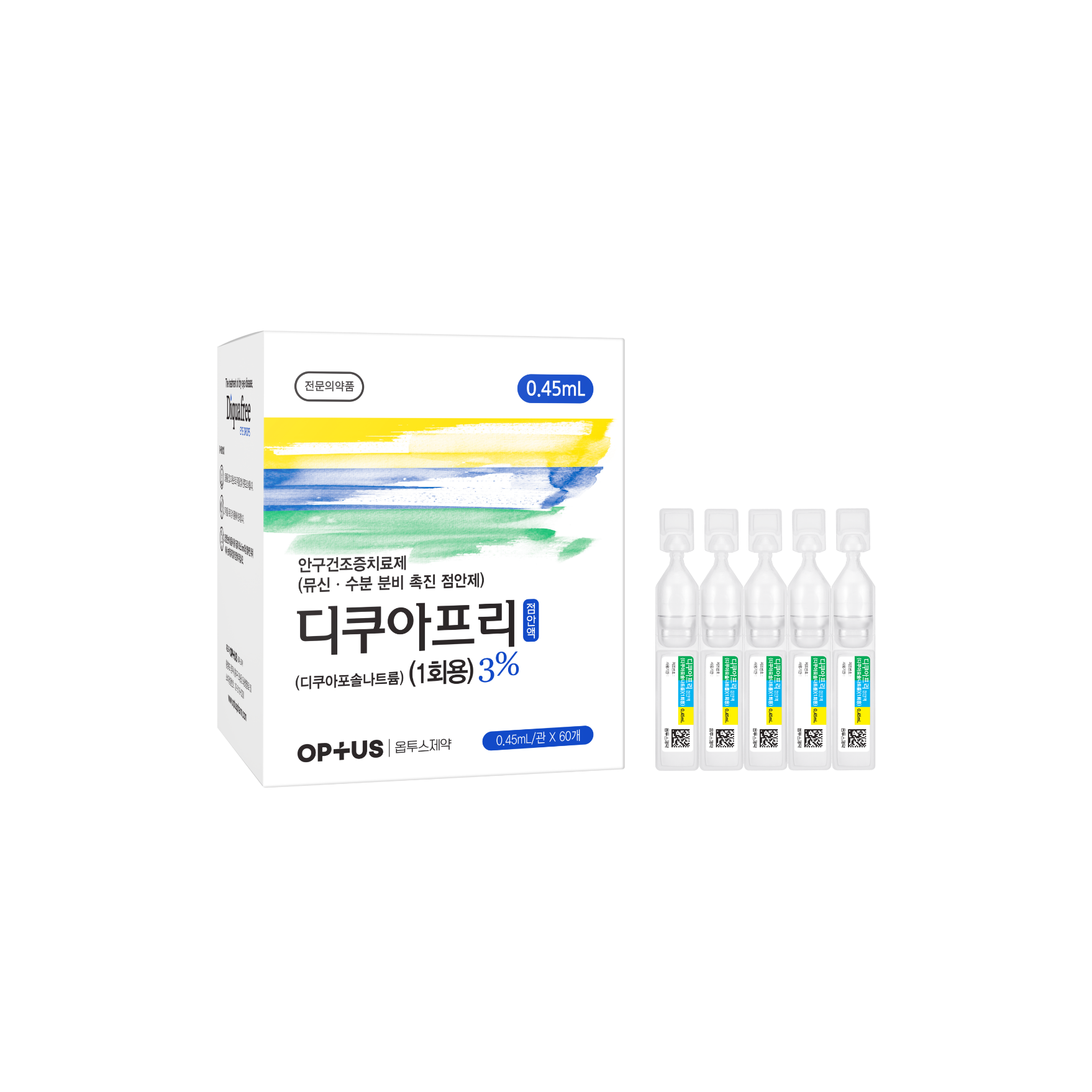
Preservative-free dry eye treatment
(Diquafosol Tetrasodium 3%)| Classification | |
|---|---|
| Active ingredients |
Diquafosol Tetrasodium 30.0mg/mL |
| Indications | Treatment of the signs and symptoms of dry eye disease |
| Dose | One drop 6 times daily |
| Packaging Unit | 0.4mL x 60s / 0.45mL x 60s |
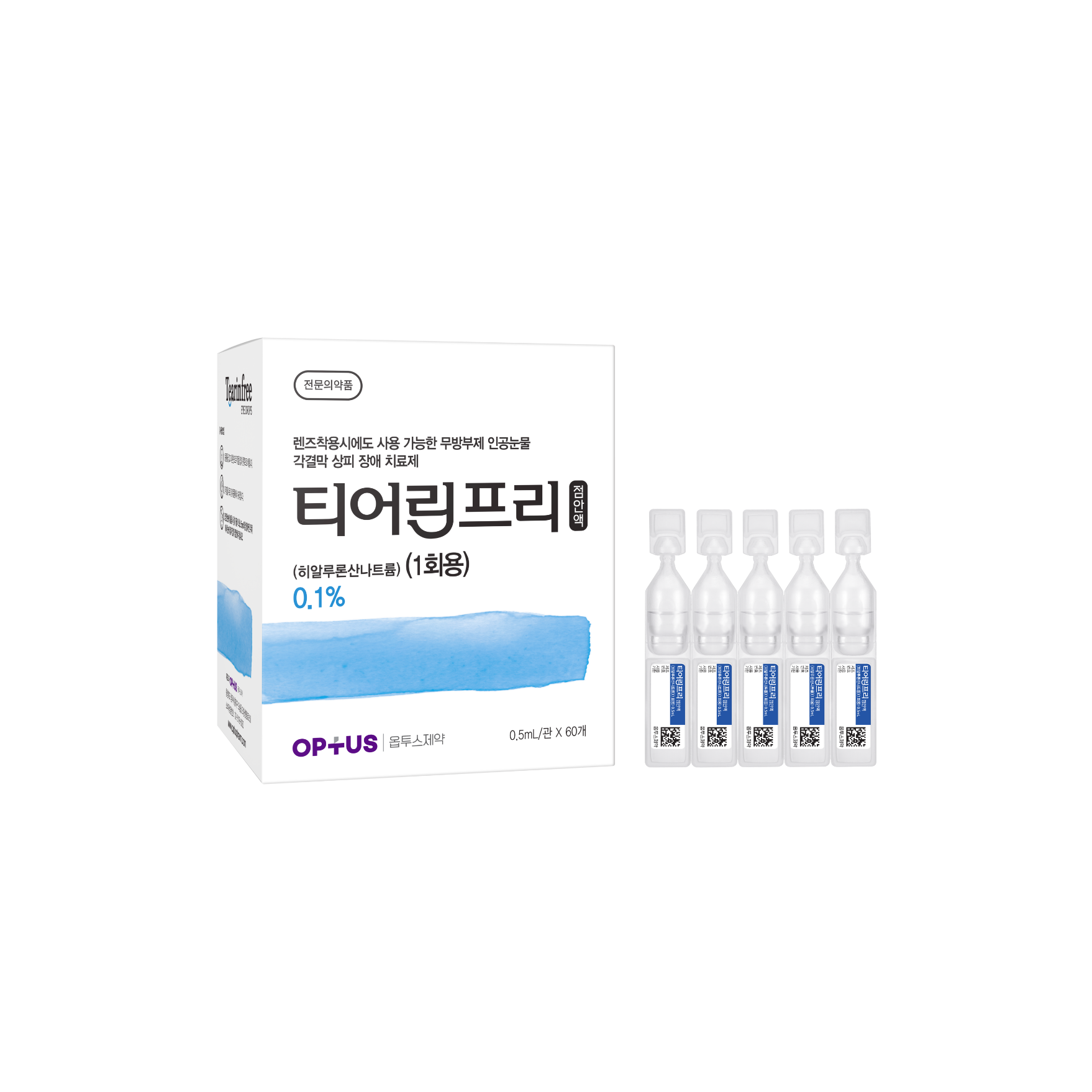
Preservative-free dry eye treatment
(Sodium Hyaluronate 0.1%)| Classification | |
|---|---|
| Active ingredients |
Sodium Hyaluronate 1.0mg/mL |
| Indications |
Epithelial disorder of cornea and conjunctiva caused by the following diseases: - Intrinsic disorders including Sjogren's syndrome, mucocutaneous ocular syndrome (Stevens-Johnson syndrome), dry eye syndrome - Extrinsic disorders caused by operation, chemical, trauma and using hard-contact lens |
| Dose | One drop 5-6 times daily |
| Packaging Unit | 0.5mL x 60s |

Dry eye treatment
(Sodium Hyaluronate 0.1%)| Classification | |
|---|---|
| Active ingredients |
Sodium Hyaluronate 1.0mg/mL |
| Indications |
Epithelial disorder of cornea and conjunctiva caused by the following diseases: - Intrinsic disorders including Sjogren's syndrome, mucocutaneous ocular syndrome (Stevens-Johnson syndrome), dry eye syndrome - Extrinsic disorders caused by operation, chemical, trauma and using hard-contact lens |
| Dose | One drop 5-6 times a day |
| Packaging Unit | 6mL x one bottle |
Preservative-free dry eye treatment
(Sodium Hyaluronate 0.15%)| Classification | |
|---|---|
| Active ingredients |
Sodium Hyaluronate 1.5mg/mL |
| Indications |
Epithelial disorder of cornea and conjunctiva caused by the following diseases: - Intrinsic disorders including Sjogren’s syndrome, mucocutaneous ocular syndrome (Stevens-Johnson syndrome), dry eye syndrome - Extrinsic disorders caused by operation, chemical, trauma and using hard-contact lens |
| Dose | One drop 5-6 times a day |
| Packaging Unit | 0.5mL x 60s |
Preservative-free dry eye treatment
(Sodium Hyaluronate 0.18%)| Classification | |
|---|---|
| Active ingredients |
Sodium Hyaluronate 1.8mg/mL |
| Indications |
Epithelial disorder of cornea and conjunctiva caused by the following diseases: - Intrinsic disorders including Sjogren’s syndrome, mucocutaneous ocular syndrome (Stevens-Johnson syndrome), dry eye syndrome - Extrinsic disorders caused by operation, chemical, trauma and using hard-contact lens |
| Dose | One drop 5-6 times a day |
| Packaging Unit | 0.5mL x 60s |
Preservative-free dry eye treatment
(Sodium Hyaluronate 0.3%)| 구분 | |
|---|---|
| Active ingredients |
Sodium Hyaluronate 3.0mg/mL |
| Indications |
Epithelial disorder of cornea and conjunctiva caused by the following diseases: - Intrinsic disorders including Sjogren’s syndrome, mucocutaneous ocular syndrome (Stevens-Johnson syndrome), dry eye syndrome - Extrinsic disorders caused by operation, chemical, trauma and using hard-contact lens |
| Dose | One drop 5-6 times a day |
| Packaging Unit | 0.5mL x 60s |
Preservative-free dry eye treatment
(Cyclosporine 0.05%)| Classification | |
|---|---|
| Active ingredients |
Cyclosporine 0.5mg/mL |
| Indications | The increase of tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca |
| Dose | One drop 2 times a day |
| Packaging Unit | 0.4mL x 30s |
Preservative-free lubricant eye drops
(Carboxymethyl cellulose 0.5%)| Classification | |
|---|---|
| Active ingredients |
Carboxymethyl Cellulose 5.0mg/mL |
| Indications | Prevent and temporarily relieves irritation, discomfort, and burning symptoms caused by dry eyes or exposure to wind · sun |
| Dose | Fully bend the end of the container back and forth and pull it open. Instill 1-2 drops in the affected eye as needed and discard the remaining content and container. |
| Packaging Unit | 0.5mL x 30s |
Broad-spectrum antibacterial treatment
(Levofloxacin Hydrate 0.5%)| Classification | |
|---|---|
| Active ingredients |
Levofloxacin Hydrate 5.0mg/mL |
| Indications | Blepharitis, dacryocystitis, hordeolum, conjunctivitis, meibomianitis, keratitis, corneal ulcer, sterilization before/after ocular surgery |
| Dose | One drop 3 times a day |
| Packaging Unit | 6mL x one bottle |
Antibacterial treatment
(Tobramycin)| Classification | |
|---|---|
| Active ingredients |
Tobramycin 3.0mg/mL |
| Indications | blepharitis, dacryocystitis, hordeolum, conjunctivitis, keratitis, corneal ulcer |
| Dose |
1-2 drops every 4 hours, In severe cases, instill 2 drops every hour, gradually reducing the dose |
| Packaging Unit | 6mL x one bottle |

Preservative-free 4th generation quinolone antibacterial system
(Moxifloxacin Hydrochloride)| Classification | |
|---|---|
| Active ingredients |
Moxifloxacin Hydrochloride 5.45mg/mL |
| Indications | Corynebacterium species, Micrococcus luteus, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, Staphylococcus warneri, Streptococcus pneumoniae, Streptococcus viridans group, Acinetobacter lwoffii, Haemophilus influenzae, Haemophilus parainfluenzae, Chlamydia trachomatis |
| Dose |
·Inflammatory defect, sarcoiditis (including corneal ulcer): One drop 3 times a day ·Asceptic treatment before and after ophthalmic surgery: Prior to ophthalmic surgery, one drop five times daily. Following ophthalmic surgery, one drop three times daily |
| Packaging Unit | 0.4mL x 12s |

Preservative-free 4th generation quinolone antibacterial system
(Moxifloxacin Hydrochloride)| Classification | |
|---|---|
| Active ingredients |
Moxifloxacin Hydrochloride 5.45mg/mL |
| Indications | Corynebacterium species, Micrococcus luteus, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, Staphylococcus warneri, Streptococcus pneumoniae, Streptococcus viridans group, Acinetobacter lwoffii, Haemophilus influenzae, Haemophilus parainfluenzae, Chlamydia trachomatis |
| Dose |
·Inflammatory defect, sarcoiditis (including corneal ulcer): One drop 3 times a day ·Asceptic treatment before and after ophthalmic surgery: Prior to ophthalmic surgery, one drop five times a day. Following ophthalmic surgery, one drop three times a day |
| Packaging Unit | 5mL x one bottle |

Allergic conjunctivitis treatment
(Olopatadine Hydrochloride 0.1%)| Classification | |
|---|---|
| Active ingredients |
Olopatadine HCI 1.11mg/mL |
| Indications | Treatment of symptoms caused by allergic conjunctivitis |
| Dose | One drop 2 times a day |
| Packaging Unit | 6mL x one bottle |
Preservative-free allergic conjunctivitis treatment
(Olopatadine Hydrochloride 0.1%)| Classification | |
|---|---|
| Active ingredients |
Olopatadine HCI 1.11mg/mL |
| Indications | Treatment of symptoms caused by allergic conjunctivitis |
| Dose | One drop 2 times a day |
| Packaging Unit | 0.3mL x 12s |
Preservative-free allergic conjunctivitis treatment
(Olopatadine Hydrochloride 0.2%)| Classification | |
|---|---|
| Active ingredients |
Olopatadine HCI 2.22mg/mL |
| Indications | Treatment of symptoms caused by allergic conjunctivitis |
| Dose | One drop once a day |
| Packaging Unit | 0.3mL x 12s |
Preservative-free allergic conjunctivitis treatment
(Olopatadine Hydrochloride 0.7%)| Classification | |
|---|---|
| Active ingredients |
Olopatadine HCI 7.76mg/mL |
| Indications | Treatment of symptoms caused by allergic conjunctivitis |
| Dose | One drop once a day |
| Packaging Unit | 0.3mL x 12s |
Preservative-free allergic conjunctivitis treatment
(Ketotifen Fumarate)| Classification | |
|---|---|
| Active ingredients |
Ketotifen Fumarate 0.69mg/mL |
| Indications | Treatment of symptoms caused by allergic conjunctivitis |
| Dose | One drop 2-4 times a day |
| Packaging Unit | 0.45mL x 30s |

Anti-inflammatory eye disease treatment
(Fluorometholone 0.1%)| Classification | |
|---|---|
| Active ingredients |
Fluorometholone 1.0mg/mL |
| Indications | Inflammatory diseases of external segment of the eye (blepharitis, conjunctivitis, keratitis, sclerotitis, episcleritis, etc) |
| Dose | 1-2 drops 2-4 times a day |
| Packaging Unit | 6mL x one bottle |
Preservative-free glaucoma treatment
(Dorzolamide Hydrochloride, Timolol Maleate)| Classification | |
|---|---|
| Active ingredients |
Dorzolamide HCI Timolol Maleate 22.26mg/mL 6.83mg/mL |
| Indications | Reduction of intraocular pressure of the patients with open-angle glaucoma or with ocular hypertension who are insufficiently responsive to topical beta blockers. |
| Dose | One drop 2 times a day |
| Packaging Unit | 0.25mL x 30s |

Preservative-free lubricant eye drops
(Carboxymethyl cellulose 1.0%)| Classification | |
|---|---|
| Active ingredients |
Carboxymethyl Cellulose 10mg/mL |
| Indications | Temporarily relieves irritation, discomfort, and burning symptoms caused by dry eyes or exposure to wind · sun |
| Dose | Instill 1-2 drops in the affected eye as needed and discard the remaining content and container |
| Packaging Unit | 0.45mL x 60s |

Preservative-free Lubricant Eye drops
(Carboxymethyl cellulose 1.0%)| Classification | |
|---|---|
| Active ingredients |
Carboxymethyl Cellulose 10.0mg/mL |
| Indications | Temporarily relieves irritation, discomfort, and burning symptoms caused by dry eyes or exposure to wind · sun |
| Dose | Instill 1-2 drops in the eye with symptoms when necessary and discard the remaining content and container |
| Packaging Unit | 0.5mL x 60s |

Diabetic retinopathy treatment
(Dobesilate Calcium Hydrate)| Classification | |
|---|---|
| Active ingredients |
Dobesilate Calcium Hydrate 500.0mg |
| Indications | Used for preventing the progression of diabetic retinopathy(damage to the blood vessels in the retina caused by diabetes also known as diabetic eye disease) |
| Dose |
Vascular damage and encephalopathy: 500 to 1,000 mg per day Liver disease: 750 mg per day for 1 to 3 weeks as it is. 500 mg per day thereafter |
| Packaging Unit | 30 tablets/bottle, 90 tablets/bottle |

Preservative-free Lubricant Eye drops
(Carboxymethyl cellulose 0.5%)| Classification | |
|---|---|
| Active ingredients |
Carboxymethyl cellulose 5mg/mL |
| Indications | Prevent and temporarily relieves irritation, discomfort, and burning symptoms caused by dry eyes or exposure to wind · sun |
| Dose | Fully bend the end of the container back and forth and pull it open. Instill 1-2 drops in the eye with symptoms when necessary and discard the remaining content and container. |
| Packaging Unit | 0.45mL x 30s, 60s |

Preservative-free Lubricant Eye drops
| Classification | |
|---|---|
| Active ingredients |
Active ingredients in 1mL NaCl 5.5mg/mL KCl 1.5mg/mL Glucose 0.05mg/mL |
| Indications | Discomfort when wearing soft or hard contact lenses, replenish tears (dry eyes), eye fatigue, blurred eyes (eye booger) |
| Dose | one-time 2-3 drops, one day 5-6 times drops |
| Packaging Unit | 0.5mL x 30s |

Eyelid Cleaning Tissue
| Classification | |
|---|---|
| Active ingredients |
Water, Dipropylene Glycol, Glycerin, 1,2-Hexanediol, Allantoin, Butylene Glycol, Ethylhexylglycerin, Trehalose, Dipotassium Glycyrrhizate, Sodium Citrate, Euphrasia Officinalis Extract, Sodium Hyaluronate, Centella Asiatica Extract, Hippophae Rhamnoides Extract, Tocopherol |
| Indications | Eyelid Cleaner |
| Dose | After opening, gently wipe the upper and lower eyelashes and the surface of the eyelid from the inside to the outside. |
| Packaging Unit | 3mL X 15 pouches (2 cleaners per pouch) |

Eye Drop Aid
| Classification | |
|---|---|
| Active ingredients |
- |
| Indications |
Reduces the amount of eye drops being wasted by easily and accurately inserting eye drops Prevents corneal injury by keeping the distance between the eye drop container and the eye Maintains the tip of eye drop container clean, reducing the risk of infection Small in size, portable, easy to use Available for both multi-use and single-use eye drops |
| Dose |
[Multi-Use] Open the cap and insert the eye drop from aside. Tilt head up to 90° degrees, hold with ‘OUESOO’ logo facing upward, instill while looking through the focusing hole. [Single-Use] Open the lid and insert the eye drop from aside or insert the eye drop from the top with the lid closed. Tilt head up to 90° degrees, hold with ‘OUESOO’ logo facing upward, instill while looking through the focusing hole. |
| Packaging Unit | 1 EA |
Preservative-free Lubricant Eye drops
(Trehalose Hydrate )| Classification | |
|---|---|
| Active ingredients |
Trehalose Hydrate 33.16mg/mL |
| Indications | Use for dry eyes (unpleasantness, stabbing pain, irritation) or eye fatigue caused by following factors : wind, smoke, pollution, dust, dry heat, air conditioner, air travel, long hours of computer use |
| Dose | Instill 1 drop in each eye several times a day when necessary. Discard the remaining content and container. |
| Packaging Unit | 0.5mL x 30s, 60s |

Preservative-free Lubricant Eye drops
| Classification | |
|---|---|
| Active ingredients |
Active ingredients in 1mL Chondroitin Sulfate Sodium 5mg/mL Hypromellose 2910 3mg/mL NaCl 4.4mg/mL KCl 0.8mg/mL |
| Indications | Eye fatigue, replenish tears(dry eyes), Discomfort when wearing soft or hard contact lenses, blurred eyes(eye booger) |
| Dose | One-time 2-3 drops, one day 4-5 times drops |
| Packaging Unit | 0.5mL x 30s |

Micro Needle Eye Patch
| Classification | |
|---|---|
| Active ingredients |
Sodium Hyaluronate, Glyceryl Caprylate, Xylitol, Hydrolyzed Hyaluronic Acid, Pinus Densiflora Leaf Extract, Artemisia Princeps Leaf Extract, Sophora Flavescens Root Extract, Laminaria Digitata Extract, Leontopodium Alpinum Callus Culture Extract, Acetyl Hexapeptide-8, Copper Tripeptide-1, Oligopeptide-29, Oligopeptide-32, Palmitoyl Pentapeptide-4 |
| Indications |
Micro needle provides vitality and energy with professional delicate touch to the tired eyes from non-stop screen time by delivering Active ingredients effectively |
| Dose | Apply and gently press the patches to the desired spot to attach closely to the skin. Remove the patches after at least 1 hour |
| Packaging Unit | 2 patches/pair X 3 pouches |

Hydrogel Eye Patch
| Classification | |
|---|---|
| Active ingredients |
Water, Glycerin, Sorbitol, Sodium Polyacrylate, Cellulose Gum, Kaolin, Sodium Hyaluronate, Sodium Acetylated Hyaluronate, Hydrolyzed Hyaluronic Acid, Laminaria Digitata Extract, Menthyl Lactate, Portulaca Oleracea Extract, Arginine, Tocopheryl Acetate, Polyacrylic Acid, Disodium EDTA, Allantoin, Aluminum Glycinate, Tartaric Acid, Triethylhexanoin, Ricinus Communis Seed Oil, 1,2-Hexanediol, Titanium Dioxide |
| Indications | Excellent moisture and nutrition providing gel patch with high adhesion, making healthy and elastic skin around the eyes. Cooling gel with astringent and calming effect makes clear and clean skin |
| Dose | Apply the patches to the desired spot and remove the patches after 20~30 minutes |
| Packaging Unit | 2g(1g X 2patches)/pair X 5 pouches |

Eye Health · Eyestrain Improvement
| Classification | Health Functional Food |
|---|---|
| Active ingredients |
Marigold Extract, Haematococcus Extract, Soybean Oil, Rice Bran Wax, Soybean Lecithin, Candelilla Wax, Vitamin A, Vitamin C, d-α-Tocopherol, Zinc Oxide, Cupric Sulphate, Bilberry Extract, Goji Berry Extract, Acai Berry Extract, Hyaluronic Acid *Capsule Components: Food Starch Modified, Glycerin, Carrageenan, D-Sorbitol Solution, Annatto Extract, Gardenia Blue Contains soybean and peanut Amount per serving: 0 kcal, Carbohydrate 0 g(0 %), Protein 0 g(0 %), Fat 0 g(0 %), Sodium 0 mg(0 %), Lutein 20 mg, Astaxanthin 6 mg ※ ( ) % daily value |
| Indications |
[Marigold Extract] May support eye health by maintaining the density of macular pigments which can be affected by aging [Haematococcus Extract] May help to reduce eyestrain |
| Directions | 1 capsule daily |
| Packaging Unit | 400 mg X 30 Capsules |
Digital Eyestrain Improvement
| Classification | Health Functional Food |
|---|---|
| Active ingredients |
Red Perilla Extract, Microcrystalline Cellulose, Hydroxypropylmethyl Cellulose, Magnesium Stearate, Silicon Dioxide, Glycerin Fatty Acid Esters, Titanium Dioxide, Lac Color, Gardenia Blue, Bilberry Extract, Goji Berry Extract, Acai Berry Extract, Hyaluronic Acid Amount per serving: 0 kcal, Carbohydrate 0 g(0 %), Protein 0 g(0 %), Fat 0 g(0 %), Sodium 0 mg(0 %), Luteolin-7-O-diglucuronide 22.3 mg ※ ( )% daily value |
| Indications | May help to reduce eyestrain |
| Directions | 1 tablet daily |
| Packaging Unit | 600 mg X 15 Tablets X 2 ea |

Eye Health Supplement
| Classification | Health Functional Food |
|---|---|
| Active ingredients |
Marigold Extract, Starch Syrup, Sugar, Purified Water, Gelatin,
Citric Acid, Flavoring Substances, Pectin, Processed Edible Oil Product, Black Carrot Juice Concentrate, Enzymatically Modified Stevia, Trisodium Citrate, Glycerin Esters of Fatty Acids, Hyaluronic Acid, Fructooligosaccharide, Bilberry Extract Powder, Vitamin A, Zinc Oxide, Carrot Juice Powder, Seven Berry Extract, Blueberry Extract, Vitamin D₃ Contains pork and soybean Amount per serving: 30 kcal, Carbohydrate 7 g(2 %), Total Sugars 5 g(5 %), Protein 1 g(2 %), Fat 0 g(0 %), Sodium 0 mg(0 %), Lutein 10 mg ※ ( ) % daily value |
| Indications | May support eye health by maintaining the density of macular pigments which can be affected by aging |
| Directions | 3 gummies daily |
| Packaging Unit | 3 g X 30 Gummies X 3 Packs |
Improvement of Dry Eyes ·
Skin Moisturizing · Antioxidant
| Classification | Health Functional Food |
|---|---|
| Active ingredients |
Low-temperature Supercritical Omega 3, Probiotics fermented Hyaluronic Acid, d-α-Tocopherol, Vitamin A, Soybean Oil, Beeswax, Soybean Lecithin, Cupric Gluconate, Vitamin C, Zinc Oxide, Manganese Sulphate, Senna Tora Extract Powder
*Capsule Components: Hydroxypropyl Starch Powder, Glycerin, Carrageenan, Polyglycitol Syrup, Gardenia Blue, Cacao Color Contains peanut and soybean Amount per serving: 15 kcal, Carbohydrate 0 g(0 %), Protein 0 g(0 %), Fat 1.5 g(3 %), Sodium 10 ㎎(1 %), Vitamin A 700 ㎍ RAE(100 %), Vitamin E 11 ㎎ α-TE(100 %), EPA+DHA 600 ㎎, Hyaluronic Acid 240 ㎎ ※ ( ) % daily value |
| Indications |
[DHA & EPA] May help to maintain eye health as the improvement of dry eyes, May help to maintain healthy triglyceride level and blood flow [Hyaluronic Acid] May help to maintain skin moisturizing, May help to maintain skin health from skin damage by UV radiation [Vitamin A] Necessary for vision adaptation in dark places, Necessary for the normal structure and function of skin and mucosa, Necessary for the normal growth and development of epithelial cells [Vitamin E] Necessary for the protection of cell from free radicals by anti-oxidant activity |
| Directions | 2 capsules daily |
| Packaging Unit | 900 mg X 60 Capsules |
Preservative-free allergic conjunctivitis treatment
(Ketotifen Fumarate )| Classification | |
|---|---|
| Active ingredients |
Ketotifen Fumarate 0.69mg/mL |
| Indications | Treatment of symptoms caused by allergic conjunctivitis |
| Dose | One-time 1drop, one day 2-4 times drops |
| Packaging Unit | 0.5mL x 12s |

Eyestrain Improvement·
Energy Production·Antioxidant
| Classification | Health Functional Food |
|---|---|
| Active ingredients |
Red Perilla Extract, D-α-Tocopheryl Acetate, Nicotinamide, Vitamin D₃, Pyridoxine Hydrochloride, Thiamine Mononitrate, Riboflavin, Purified Water, Apple Concentrate, Isomaltooligosaccharides, Guarana Extract Powder, Locust Bean Gum, Apple Flavor, γ-Cyclodextrin, Citric Acid, Taurine, Theanine, Trisodium Citrate, Carrageenan, Sucralose, Magnesium Gluconate, Vitamin A, Zinc Oxide
Contains soybean Amount per serving: 15 kcal, Carbohydrate 5 g(2 %), Total Sugars 2 g(2 %), Sodium 20 ㎎(1 %), Luteolin-7-O-diglucuronide 22.3 ㎎, Vitamin B₁ 1.2 ㎎(100 %), Vitamin B₂ 1.4 ㎎(100 %), Vitamin B₆ 1.5 ㎎(100 %), Vitamin D 10 ㎍(100 %), Vitamin E 11 ㎎ α-TE(100 %), Niacin 15 ㎎ NE(100 %) ※ ( ) % daily value |
| Indications |
[Red Perilla Extract] May help to reduce eyestrain [Vitamin B₁] Necessary for the normal carbohydrates and energy metabolism [Vitamin B₂] Necessary for the energy production in the body [Vitamin B₆ ] Necessary for the utilization of proteins and amino acids, Necessary for the maintenance of normal blood homocysteine levels [Vitamin D] Necessary for the normal absorption and utilization of calcium and phosphorus, Necessary for the normal structure and maintenance of bones, May help to reduce the risk of osteoporosis [Vitamin E] Necessary for the protection of cell from free radicals by anti-oxidant activity [Niacin] Necessary for the energy production in the body |
| Directions | 1 stick daily |
| Packaging Unit | 20 g X 7 Sticks |

Broad-spectrum antibacterial treatment
(Levofloxacin hydrate 1.5%)| Classification | |
|---|---|
| Active ingredients |
Levofloxacin Hydrate 15mg/mL |
| Indications | Blepharitis, dacryocystitis, hordeolum, conjunctivitis, meibomianitis, keratitis, corneal ulcer, sterilization before/after ocular surgery |
| Dose | One drop 3 times a day |
| Packaging Unit | 5mL x one bottle |

Blue Light Protection Eye Serum
| Classification | |
|---|---|
| Active ingredients |
Aqua (Water), Glycerin, Niacinamide, Tris-Biphenyl Triazine, Pentylene Glycol, Caesalpinia Spinosa Fruit Extract, Helianthus Annuuss (Sunflower) Sprout Extract, Albizia Julibrissin Bark Extract, Sigesbeckia Orientalis Extract, Sodium Hyaluronate, Adenosine, Sodium Ascorbyl Phosphate, Ascorbyl Palmitate, Tocopherol, Tocopheryl Acetate, Retinyl Palmitate, Panthenol, Phosphatidylcholine, Lecithin, Chondrus Crispus (Carrageenan), Maltodextrin, Sodium Hydroxide, Decyl Glucoside, Tetrasodium Glutamate Diacetate, Citric Acid, Carbomer, Xanthan Gum, Sodium Polyacrylate, Butylene Glycol, Propanediol, Glyceryl Caprylate, Caprylyl Glycol, Sodium Benzoate, Disodium Phosphate |
| Indications | A steel-ball-typed blue light block eye serum prevents the skin around the eyes from reactive oxygen species and provides elasticity along with whitening and anti-wrinkle effect. |
| Dose | Gently roll and let the serum absorb around the eyes at the last step of morning skin care. |
| Packaging Unit | 15ml |

Blue Light Protection Eye Cream
| Classification | |
|---|---|
| Active ingredients |
Aqua (Water), Caprylic/Capric Triglyceride, Isostearyl Isostearate, Glycerin, Niacinamide, Sucrose Stearate, Cetyl Alcohol, Maltodextrin, Pentylene Glycol, Sodium Polyacrylate, Glyceryl Stearate, Tris-Biphenyl Triazine, Butylene Glycol, Jojoba Esters, Sodium Stearoyl Glutamate, Caprylyl Glycol, Helianthus Annuus (Sunflower) Seed Wax, Panthenol, Phosphatidylcholine, Xanthan Gum, Decyl Glucoside, Sodium Ascorbyl Phosphate, Tocopheryl Acetate, Glyceryl Caprylate, Propanediol, Lecithin, Tetrasodium Glutamate Diacetate, Citric Acid, Caesalpinia Spinosa Fruit Extract, Adenosine, Acacia Decurrens Flower Wax, Dunaliella Salina Extract, Polyglycerin-3, Retinyl Palmitate, Disodium Phosphate, Helianthus Annuus (Sunflower) Sprout Extract, Palmitoyl Tripeptide-5, Sodium Hyaluronate, Tocopherol, Pantolactone, Ascorbyl Palmitate |
| Indications | A spot-typed blue light block eye cream provides brightness around the eyes by preventing the skin from blue light and providing hydration along with whitening and anti-wrinkle effect. |
| Dose | Gently spread proper amount of the product and let the cream absorb around the eyes at the last step of morning skin care. |
| Packaging Unit | 15ml |
Preservative-free glaucoma treatment
(Latanoprost)| Classification | |
|---|---|
| Active ingredients |
Latanoprost 0.05mg/mL |
| Indications | Reduction of intraocular pressure in open-angle glaucoma and ocular hypertension |
| Dose | One drop once daily |
| Packaging Unit | 0.2mL x 30s, 0.2mL x 60s |
Preservative-free broad-spectrum antibacterial treatment
(Levofloxacin hydrate 1.5%)| Classification | |
|---|---|
| Active ingredients |
Levofloxacin Hydrate 15mg/mL |
| Indications | Blepharitis, dacryocystitis, hordeolum, conjunctivitis, meibomianitis, keratitis, corneal ulcer, sterilization before/after ocular surgery |
| Dose | One drop 3 times a day |
| Packaging Unit | 0.4mL x 12s |

Lutein Zeaxanthin Complex Extract
| Classification | Health Functional Food |
|---|---|
| Active ingredients |
Lutein Zeaxanthin Complex Extract (India/Lutein Zeaxanthin Complex Extract, Sunflower Oil) Lutein 18.18mg, Total Zeaxanthin 1.82mg ※ Capsule Components: Modified Food Starch, Glycerin, Carrageenan, Polyglycitol Syrup, Cacao Pigment |
| Indications | [Lutein Zeaxanthin Complex Extract (No. 2018-4)] May support eye health by maintaining the density of macular pigments which can be affected by aging |
| Directions | Take 1 capsule per day with sufficient amount of water |
| Packaging Unit | 90.91 mg x 30 Capsules (2.7273 g) |