

All products
Dry eye preparation
antibiotics
anti-allergic preparations
Steroids preparation
Glaucoma treatment
retinopathy treatment
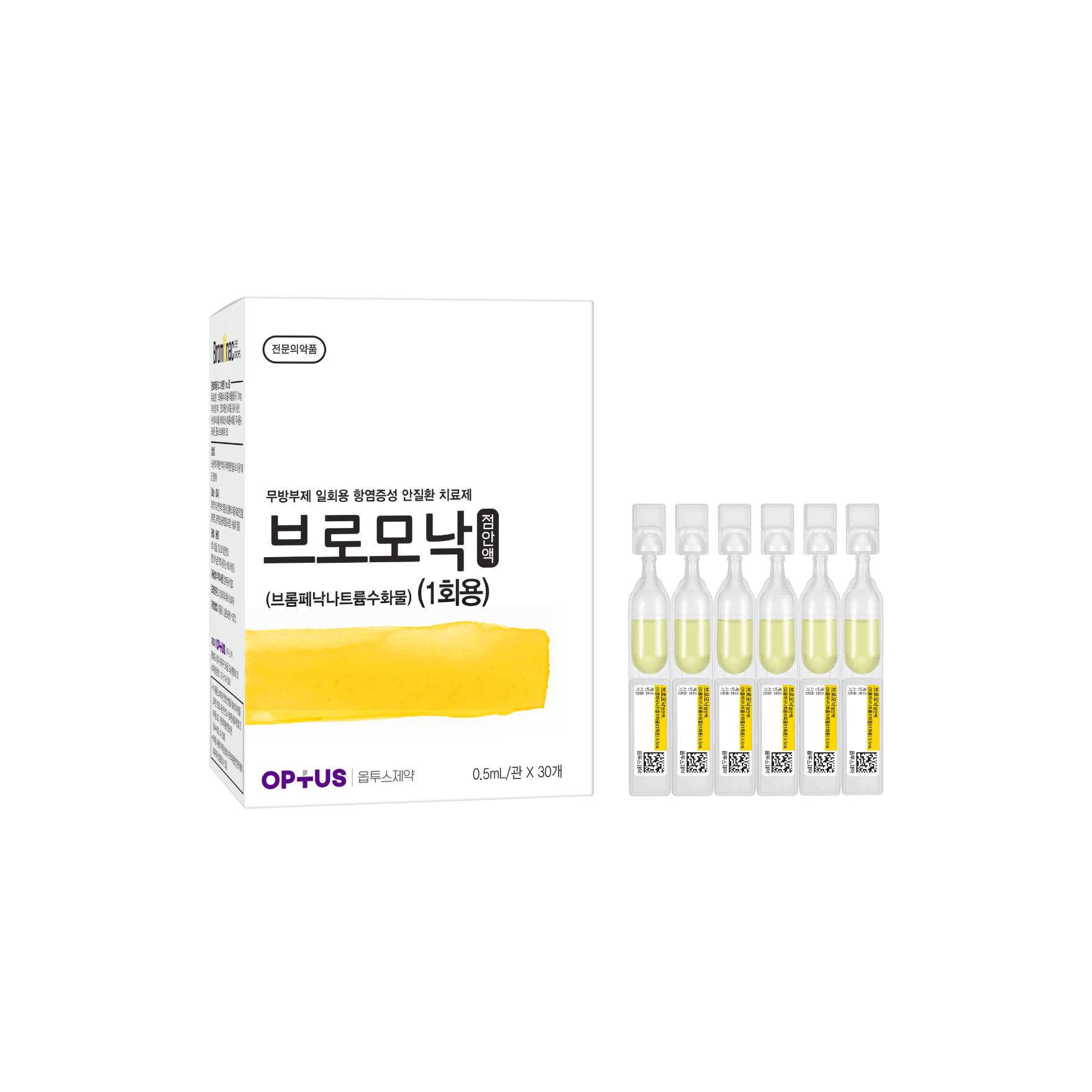
Preservative-free NSAID eye disease treatment
(Bromfenac Sodium Hydrate)| Classification | |
|---|---|
| Active ingredients |
Bromfenac Sodium Hydrate 1.0mg/mL |
| Indications |
Inflammatory diseases of the outer and anterior segment of the eye (blepharitis, conjunctivitis, scleritis (inclUnit Doseing episcleritis), postoperative inflammation) |
| Dose | One drop twice daily |
| Packaging Unit | 0.5mL x 30s |
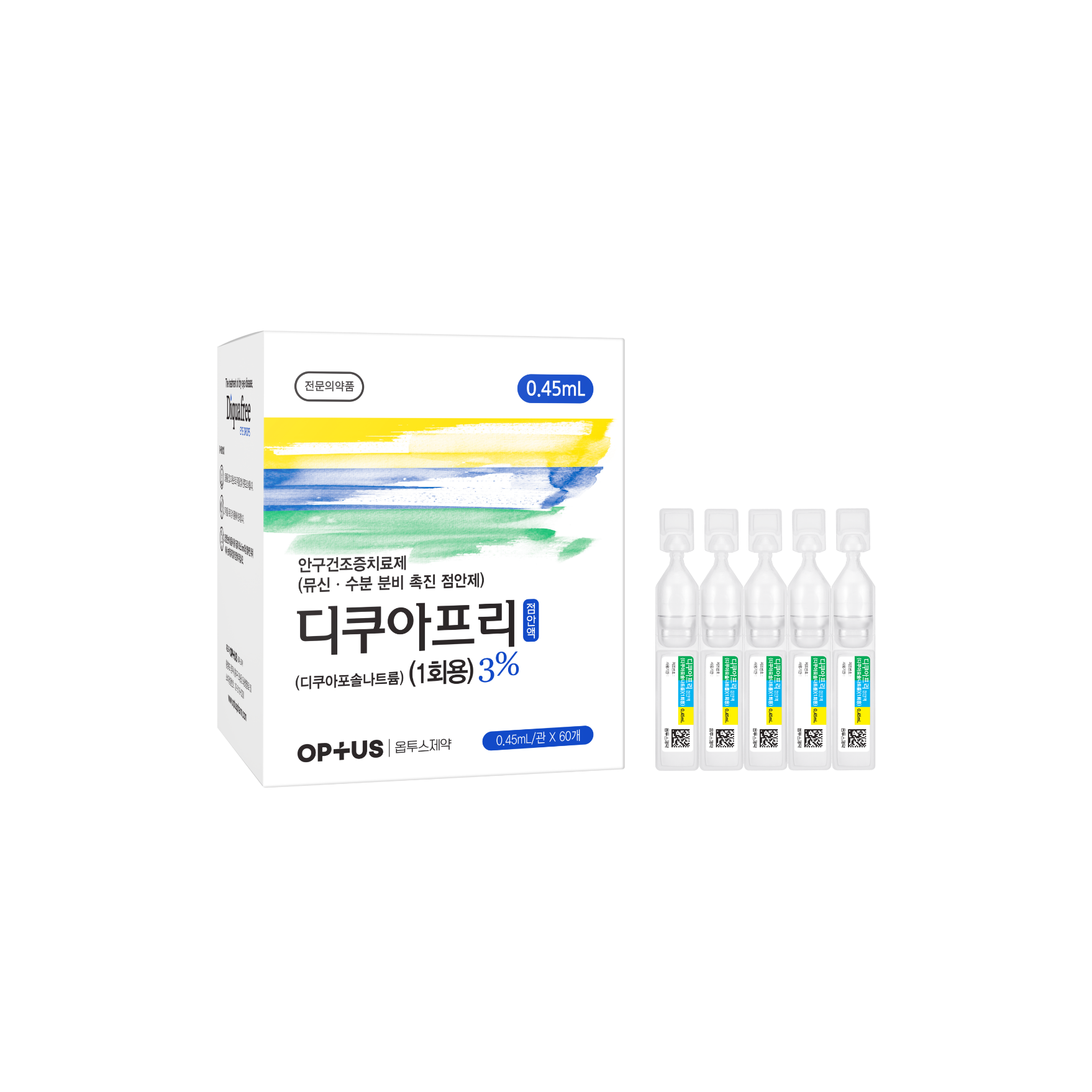
Preservative-free dry eye treatment
(Diquafosol Tetrasodium 3%)| Classification | |
|---|---|
| Active ingredients |
Diquafosol Tetrasodium 30.0mg/mL |
| Indications | Treatment of the signs and symptoms of dry eye disease |
| Dose | One drop 6 times daily |
| Packaging Unit | 0.4mL x 60s / 0.45mL x 60s |
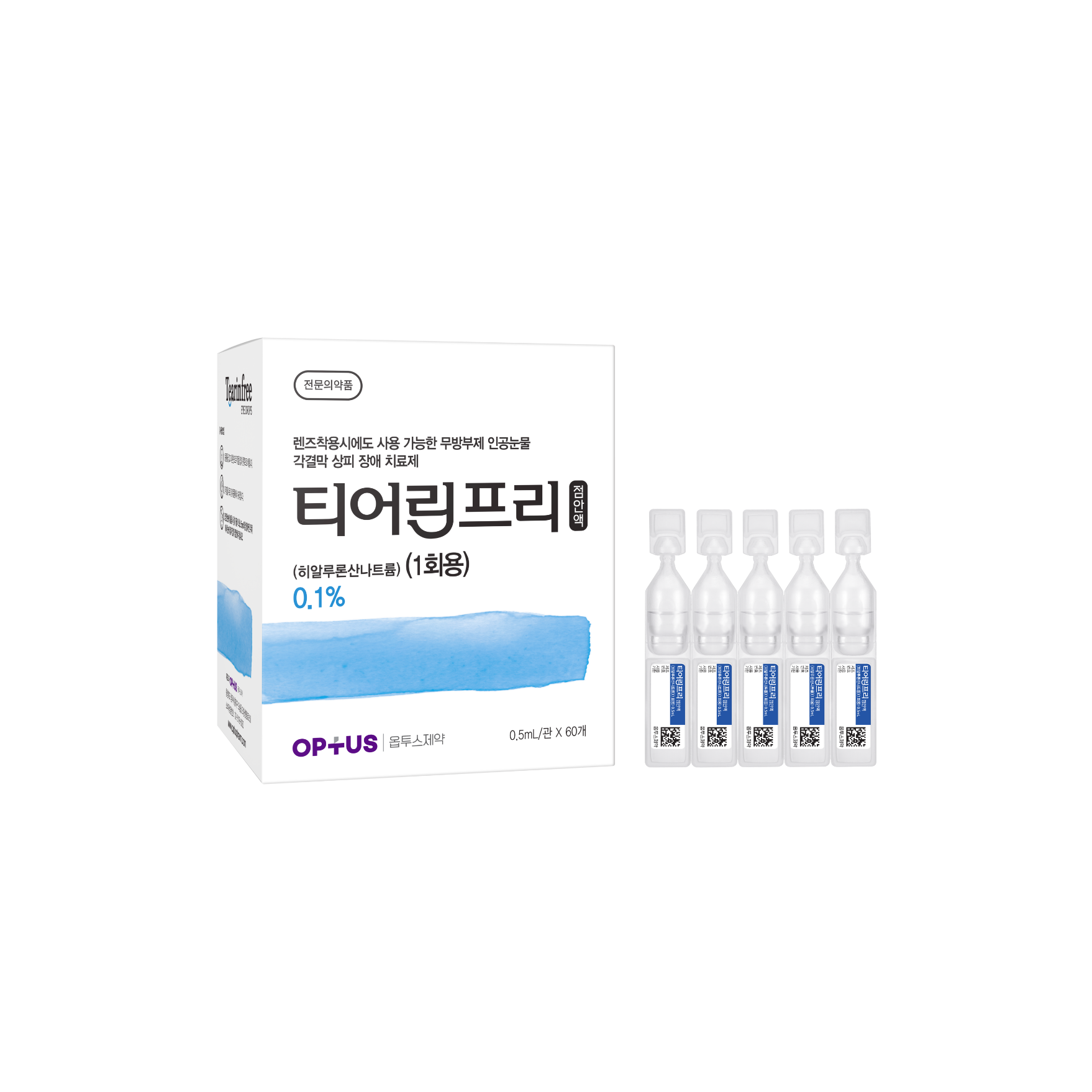
Preservative-free dry eye treatment
(Sodium Hyaluronate 0.1%)| Classification | |
|---|---|
| Active ingredients |
Sodium Hyaluronate 1.0mg/mL |
| Indications |
Epithelial disorder of cornea and conjunctiva caused by the following diseases: - Intrinsic disorders including Sjogren's syndrome, mucocutaneous ocular syndrome (Stevens-Johnson syndrome), dry eye syndrome - Extrinsic disorders caused by operation, chemical, trauma and using hard-contact lens |
| Dose | One drop 5-6 times daily |
| Packaging Unit | 0.5mL x 60s |

Dry eye treatment
(Sodium Hyaluronate 0.1%)| Classification | |
|---|---|
| Active ingredients |
Sodium Hyaluronate 1.0mg/mL |
| Indications |
Epithelial disorder of cornea and conjunctiva caused by the following diseases: - Intrinsic disorders including Sjogren's syndrome, mucocutaneous ocular syndrome (Stevens-Johnson syndrome), dry eye syndrome - Extrinsic disorders caused by operation, chemical, trauma and using hard-contact lens |
| Dose | One drop 5-6 times a day |
| Packaging Unit | 6mL x one bottle |
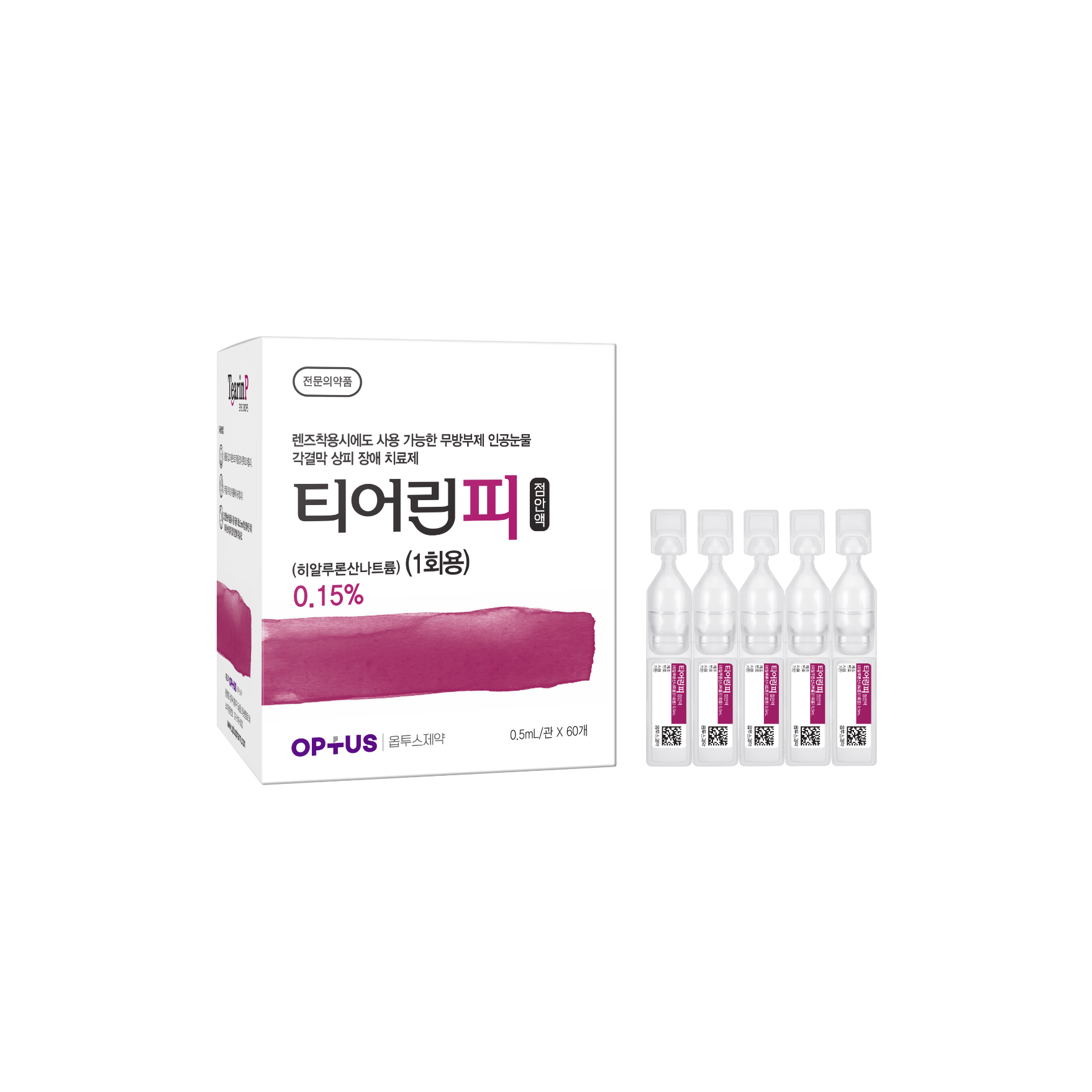
Preservative-free dry eye treatment
(Sodium Hyaluronate 0.15%)| Classification | |
|---|---|
| Active ingredients |
Sodium Hyaluronate 1.5mg/mL |
| Indications |
Epithelial disorder of cornea and conjunctiva caused by the following diseases: - Intrinsic disorders including Sjogren’s syndrome, mucocutaneous ocular syndrome (Stevens-Johnson syndrome), dry eye syndrome - Extrinsic disorders caused by operation, chemical, trauma and using hard-contact lens |
| Dose | One drop 5-6 times a day |
| Packaging Unit | 0.5mL x 60s |
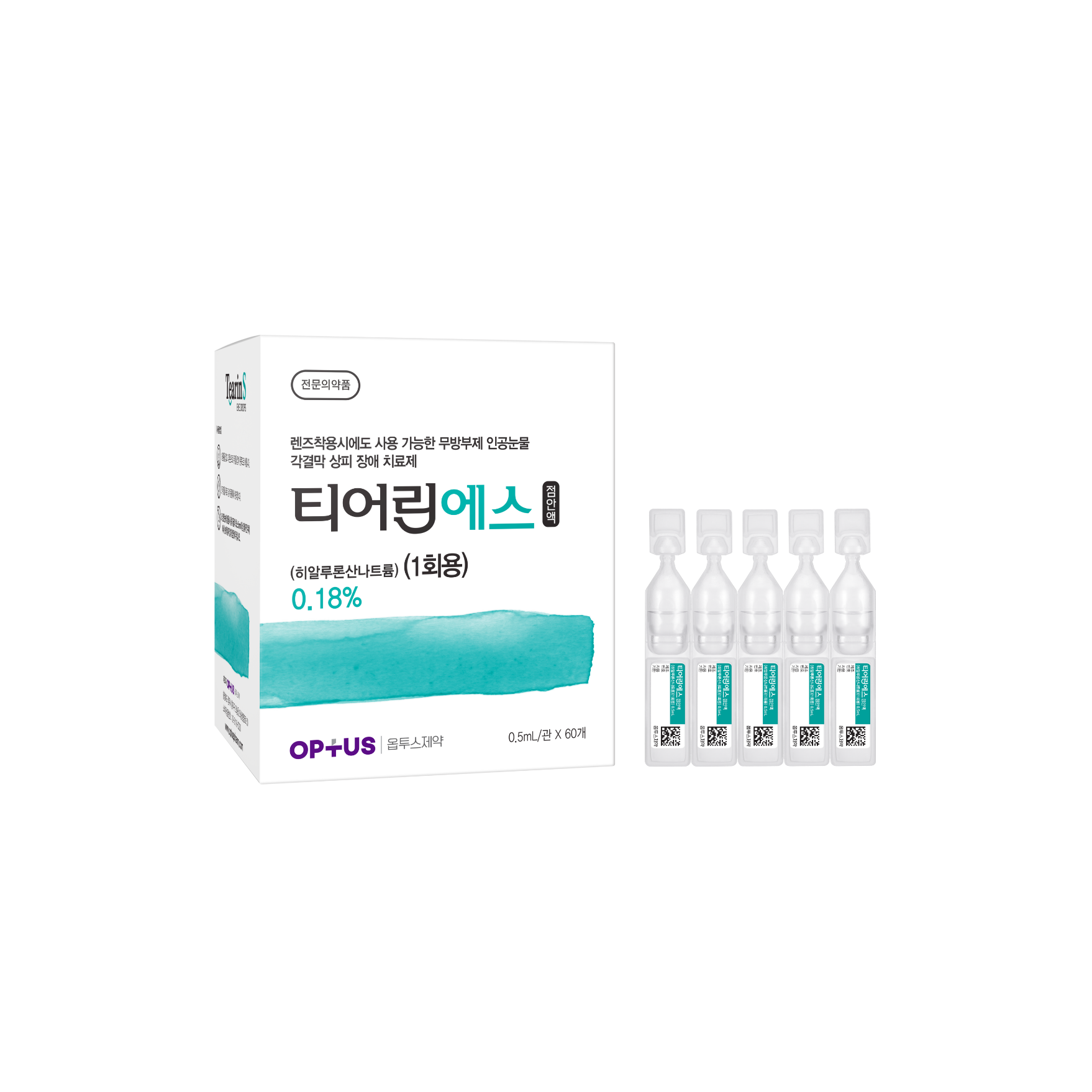
Preservative-free dry eye treatment
(Sodium Hyaluronate 0.18%)| Classification | |
|---|---|
| Active ingredients |
Sodium Hyaluronate 1.8mg/mL |
| Indications |
Epithelial disorder of cornea and conjunctiva caused by the following diseases: - Intrinsic disorders including Sjogren’s syndrome, mucocutaneous ocular syndrome (Stevens-Johnson syndrome), dry eye syndrome - Extrinsic disorders caused by operation, chemical, trauma and using hard-contact lens |
| Dose | One drop 5-6 times a day |
| Packaging Unit | 0.5mL x 60s |
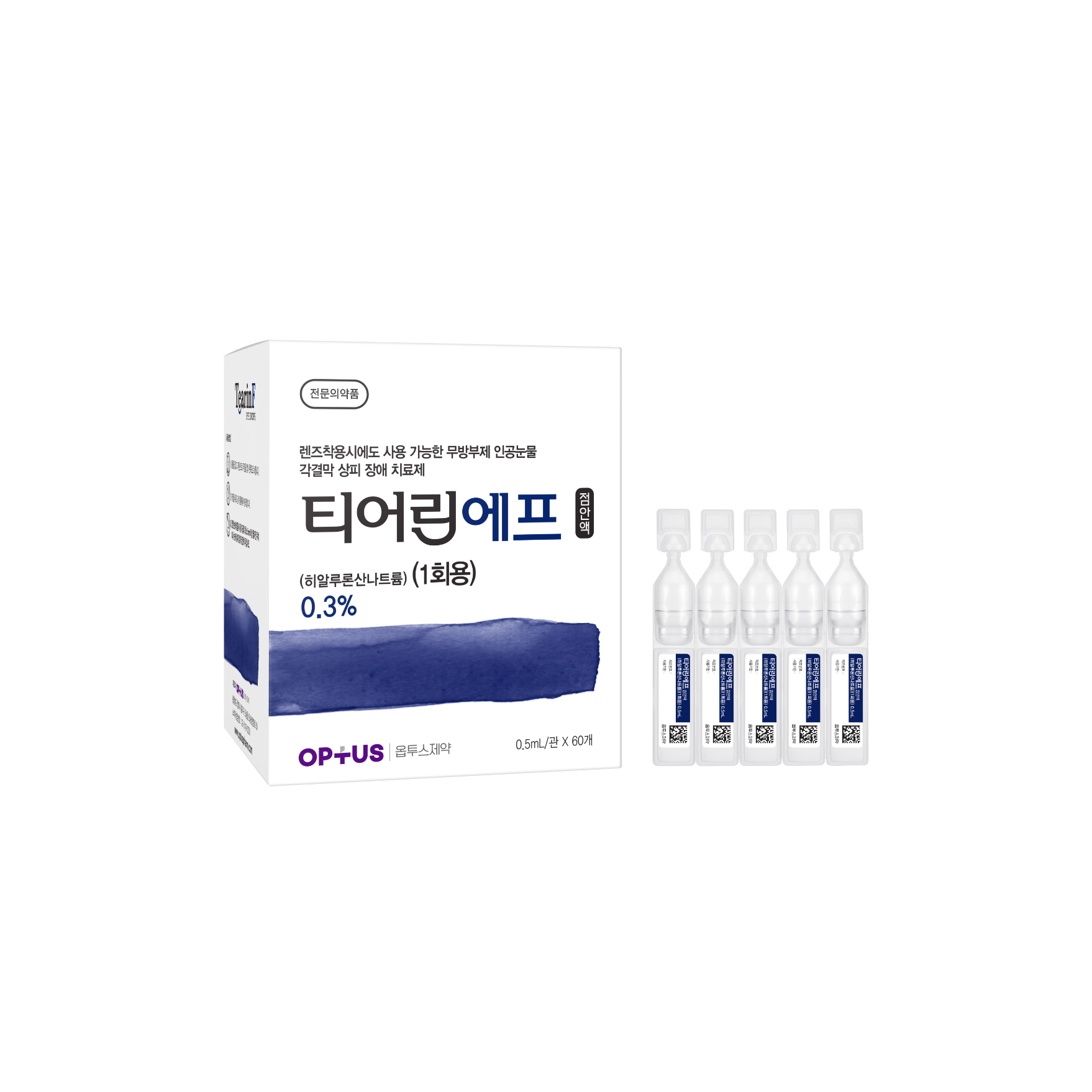
Preservative-free dry eye treatment
(Sodium Hyaluronate 0.3%)| Classification | |
|---|---|
| Active ingredients |
Sodium Hyaluronate 3.0mg/mL |
| Indications |
Epithelial disorder of cornea and conjunctiva caused by the following diseases: - Intrinsic disorders including Sjogren’s syndrome, mucocutaneous ocular syndrome (Stevens-Johnson syndrome), dry eye syndrome - Extrinsic disorders caused by operation, chemical, trauma and using hard-contact lens |
| Dose | One drop 5-6 times a day |
| Packaging Unit | 0.5mL x 60s |
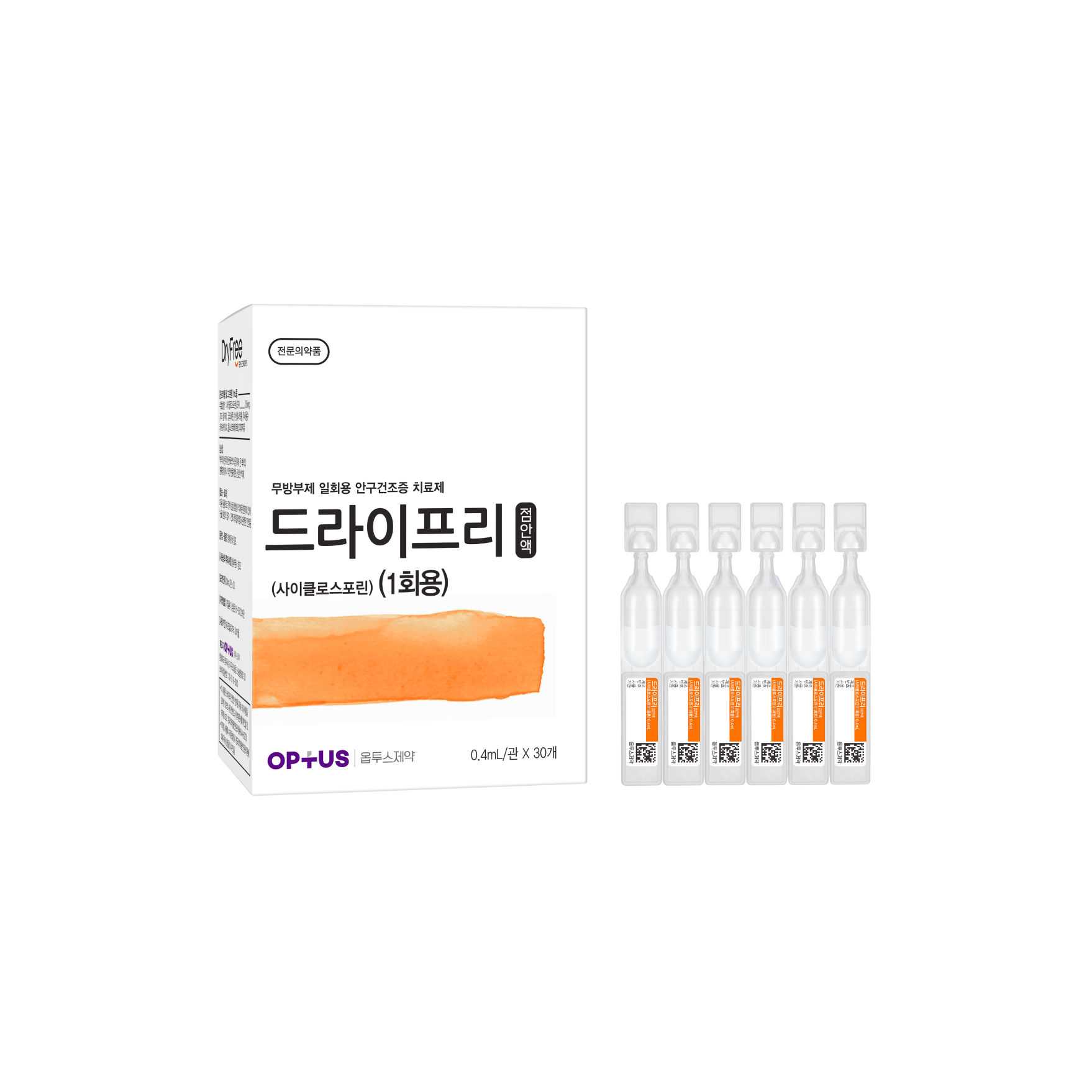
Preservative-free dry eye treatment
(Cyclosporine 0.05%)| Classification | |
|---|---|
| Active ingredients |
Cyclosporine 0.5mg/mL |
| Indications | The increase of tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca |
| Dose | One drop 2 times a day |
| Packaging Unit | 0.4mL x 30s |

Anti-inflammatory eye disease treatment
(Fluorometholone 0.1%)| Classification | |
|---|---|
| Active ingredients |
Fluorometholone 1.0mg/mL |
| Indications | Inflammatory diseases of external segment of the eye (blepharitis, conjunctivitis, keratitis, sclerotitis, episcleritis, etc) |
| Dose | 1-2 drops 2-4 times a day |
| Packaging Unit | 6mL x one bottle |
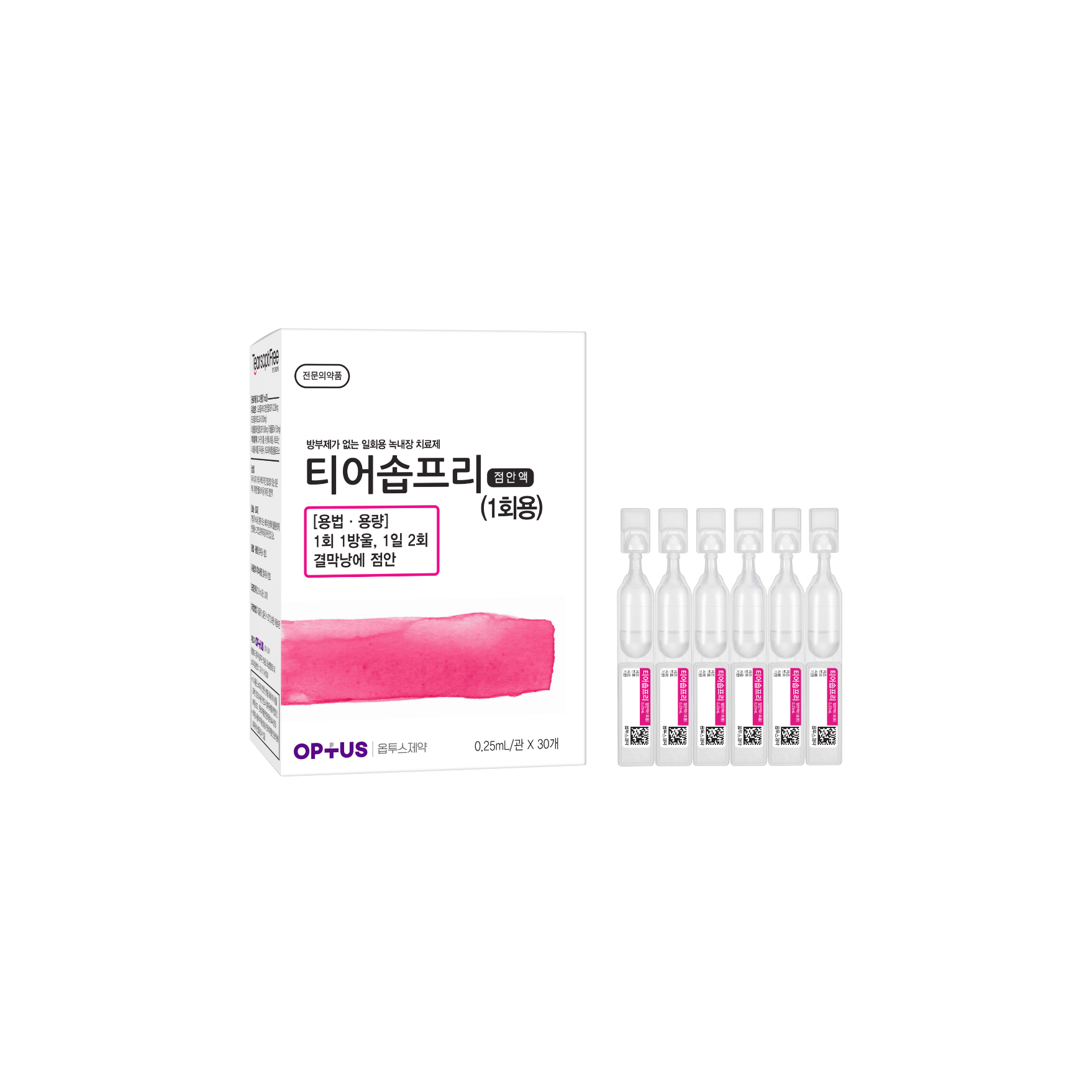
Preservative-free glaucoma treatment
(Dorzolamide Hydrochloride, Timolol Maleate)| Classification | |
|---|---|
| Active ingredients |
Dorzolamide HCI Timolol Maleate 22.26mg/mL 6.83mg/mL |
| Indications | Reduction of intraocular pressure of the patients with open-angle glaucoma or with ocular hypertension who are insufficiently responsive to topical beta blockers. |
| Dose | One drop 2 times a day |
| Packaging Unit | 0.25mL x 30s |

Diabetic retinopathy treatment
(Dobesilate Calcium Hydrate)| Classification | |
|---|---|
| Active ingredients |
Dobesilate Calcium Hydrate 500.0mg |
| Indications | Used for preventing the progression of diabetic retinopathy(damage to the blood vessels in the retina caused by diabetes also known as diabetic eye disease) |
| Dose |
Vascular damage and encephalopathy: 500 to 1,000 mg per day Liver disease: 750 mg per day for 1 to 3 weeks as it is. 500 mg per day thereafter |
| Packaging Unit | 30 tablets/bottle, 90 tablets/bottle |

Broad-spectrum antibacterial treatment
(Levofloxacin hydrate 1.5%)| Classification | |
|---|---|
| Active ingredients |
Levofloxacin Hydrate 15mg/mL |
| Indications | Blepharitis, dacryocystitis, hordeolum, conjunctivitis, meibomianitis, keratitis, corneal ulcer, sterilization before/after ocular surgery |
| Dose | One drop 3 times a day |
| Packaging Unit | 5mL x one bottle |
Preservative-free glaucoma treatment
(Latanoprost)| Classification | |
|---|---|
| Active ingredients |
Latanoprost 0.05mg/mL |
| Indications | Reduction of intraocular pressure in open-angle glaucoma and ocular hypertension |
| Dose | One drop once daily |
| Packaging Unit | 0.2mL x 30s, 0.2mL x 60s |
Preservative-free broad-spectrum antibacterial treatment
(Levofloxacin hydrate 1.5%)| Classification | |
|---|---|
| Active ingredients |
Levofloxacin Hydrate 15mg/mL |
| Indications | Blepharitis, dacryocystitis, hordeolum, conjunctivitis, meibomianitis, keratitis, corneal ulcer, sterilization before/after ocular surgery |
| Dose | One drop 3 times a day |
| Packaging Unit | 0.4mL x 12s |