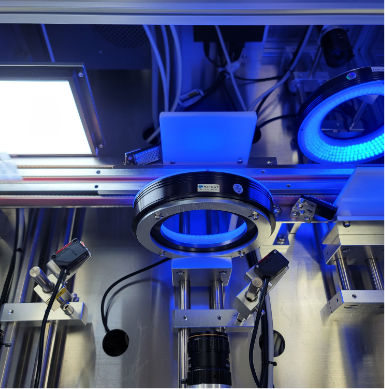
With up to date BFS technology that incorporates Blow-Fill-Seal steps, the subsequent processes, ranging from visual inspection, leak test, and packaging are streamlined and run as a single process in ways to maximize the best production efficiency.
Beginning with KGMP certification, we obtained CE & ISO 13485 certification, and EU GMP certification subsequently in 2018, OPTUS make and control products meeting the global standards

According to the through contamination control strategy, including BMS (Building Management System) monitoring system, the optimal production system is working out in clean and safe environments, free from the risks for cross-contamination.
By introduction of the fourth production line in 2022, we have the biggest production capacity in Korea for manufacture of eye drops; 470 million ampoules per year.

Planning
Annual quality control plan is established using risk management tools


Implementation
Quality control activities (e.g. product testing, validation, calibration) are performed as per the established plan


Evaluation
Comprehensive review of the carried out activities, product quality review, trend analysis, self audit


Improvement
Improvement plans for the part where improvement is considered necessary and implementation of plans.